Introduction: Clinical outcomes for non-Hispanic Black and Hispanic patients with acute myeloid leukemia (AML) have been reported to be worse compared with non-Hispanic white patients despite increasing therapy options. Socioeconomic factors play a large role in causing these disparities. However, whether specific molecular alterations are associated with a particular ethnic group is understudied in adult populations. Prior studies either involve the pediatric population or focus on a few selected ethnic groups. Our primary objective is to determine whether particular molecular alterations associated with specific ethnic groups are associated with higher mortality.
Methodology: Retrospective data was collected for 142 patients with AML diagnosed from July 2018 to December 2022 and treated in the Northwell Health system with a hypomethylating agent (HMA) and venetoclax. Demographic information was manually obtained from the electronic medical record (EMR) and ethnicity was categorized as non-Hispanic white (NHW), non-Hispanic black (NHB), Hispanic, Asian, or other/multiracial. Overall survival (OS) was calculated as the time difference between date of definitive diagnosis prior to starting venetoclax to death or date of last contact. Molecular alterations were detected by next-generation sequencing (NGS) testing. We looked at the presence of TP53, TET2, RUNX1, NPM1, FLT3-ITD, IDH1, IDH2, and several other mutations/deletions. Kaplan-Meier analysis with log-rank (Mantel-Cox) testing was used to compare overall survival between ethnic groups.
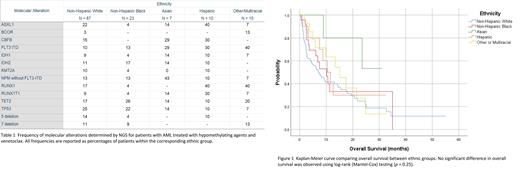
Results: Of 142 patients, 87 (61%) were NHW, 23 (16%) were NHB, 7 (5%) were Asian, 10 (7%) were Hispanic, and 15 (11%) were other/multiracial. Median age was 77 years. The most common molecular alterations were TP53 mutations/deletions (25%) for the NHW group, TET2 (27%) for the NHB group, NPM1 without FLT3-ITD (43%) for the Asian group, ASXL1 and RUNX1 (both 40%) for the Hispanic group, and RUNX1 (40%) in the other/multiracial group. The NHB group had a similar incidence of TP53 mutations/deletions (21%) as the NHW group did. Median number of cycles of HMA and venetoclax tolerated was 2 for NHW, 3 for NHB, 5 for Asians, 3 for Hispanics, and 4 for other/multiracial. Kaplan-Meier analysis comparing all groups simultaneously did not show a significant difference in OS, X 2 (4, N = 142) = 5.37, p = 0.25. Individual comparisons between the NHB (X 2 (1, N = 110) = 0.61, p = .43), Hispanic (X 2 (1, N = 97) = 0.27, p = .61), and other/multiracial (X 2 (1, N = 102) = 0.90, p = .34) groups with the NHW group did not show any significant difference either. Asians had better OS when compared individually with the NHW group (X 2 (1, N = 94) = 4.29, p = .038). However, no significant difference was observed when OS for Asians was compared with OS for any other group.
Conclusions: In this cohort of patients, the only significant OS benefit was in favor of Asians compared with NHW group. Based on molecular alterations, this may reflect the increased incidence of NPM1 mutations without FLT3-ITD mutations and decreased incidence of TP53 mutations in Asian patients. Additionally, adverse molecular alterations (TP53, RUNX1) were increased in the non-Asian groups. Asians were able to tolerate more cycles of HMA and venetoclax than any other group. Though prior studies suggested that Asians have molecular alterations with favorable risk, the sample size in this study for the Asian group (N = 7) was too small to make any definitive conclusions. Future studies should build on these findings.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal